Pfizer coronavirus vaccine receives emergency use authorization
The FDA authorized the Pfizer/BioNTech vaccine in the US; Cofepris authorized it in Mexico
The Pfizer coronavirus vaccine has been granted emergency approval by the US Food and Drug Administration (FDA) on Friday, December 11th of 2020. Most importantly, this emergency use authorization is paramount for two reasons:
- Its the first time in US history that the FDA grants emergency use authorization to a messenger RNA (mRNA) vaccine.
- This is also the first time that the FDA grants its approval to a vaccine that prevents a Covid-19 infection.
Similarly, COFEPRIS, the Mexican health regulatory agency, granted emergency use authorization to the Pfizer/BioNTech Covid-19 vaccine. Following the footsteps of the UK and Canada, Mexico became the first Latin American country to authorize its emergency use. Fortunately, this historic authorization granted by COFEPRIS will provide access to the Pfizer coronavirus vaccine to millions of vulnerable Mexicans.

The approval of the Pfizer coronavirus vaccine: a turning point in the Covid-19 pandemic
The emergency use authorization of Pfizer’s coronavirus vaccine is a light of hope for the coronavirus pandemic, which has claimed more than 300,000 lives in the US and more than 110,000 lives in Mexico.
According to Dr. Stephen Hahn, FDA commissioner: “The FDA’s authorization for emergency use of the first Covid-19 vaccine is a significant milestone in battling this devastating pandemic that has affected so many families in the United States and around the world.”
With these two authorizations granted by the FDA and Cofepris, the US and Mexico have followed the example of other countries. Specifically, the UK and Canada have authorized the use of the Pfizer/BioNTech Covid-19 vaccine for broad public use. In this sense, it is worth noting that the FDA and Cofepris approved the BNT162b2 Pfizer vaccine after a Phase III trial which involved more than 40,000 people.
The FDA and Cofepris approved the Pfizer/BioNTech Covid-19 vaccine due to successful results in its clinical trials
Most importantly, clinical trials of this vaccine demonstrated that it is effective at preventing coronavirus 95% of the time. In addition, the data showed that this vaccine is safe and has mild side effects in adults. Therefore, the FDA approved its use in people who are 16 years or older. Nonetheless, Pfizer is continuing to test its vaccine in children. Thus, volunteer children under age 16 will get the vaccine to determine its safety and efficacy among this group. However, even after its successful results and its authorization for emergency use, two challenges still lay ahead. One major obstacle is being able to produce sufficient doses of the vaccine. The other challenge is overcoming its complex distribution requirements.
The initial supply of the Pfizer coronavirus vaccine will be scarce; its distribution will be a challenge
Pfizer’s covid-19 vaccine will be in limited and short supply (at least initially)
One thing is for sure: Initially, at least during the first six months, the Pfizer/BioNTech covid-19 vaccine will be in short supply. For example, the US will only receive 3 million doses in December of 2020. Moreover, only long-term care residents and healthcare workers will receive these first vaccines. This is because the highest risk of getting a severe Covid-19 infection is most prevalent in these two groups. Subsequently, two weeks later, Pfizer will provide the US government with a second 3 million dose allotment. Accordingly, this group of people will receive their second booster shot. This way, after these two consecutive shots, the Pfizer covid-19 vaccine will achieve its desired 95% efficacy rate.
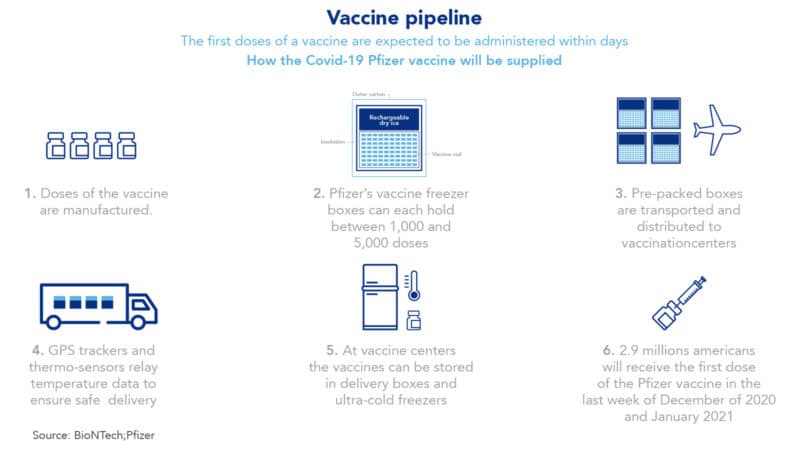
The distribution of Pfizer’s coronavirus vaccine will be a complex challenge
The Pfizer coronavirus vaccine requires a transportation temperature of -94 degrees Fahrenheit, equivalent to -70 degrees Celsius. Therefore, the storage and transportation of this novel MRNA vaccine will be a true logistical challenge. In turn, this challenge will require close coordination of entities such as the US government, transportation companies, health agencies and local hospitals. The Pfizer vaccine will be shipped in special boxes which will contain dry ice and are specifically designed to maintain an ultra-cold temperature. In addition, these boxes will include a GPS enabled tracking device that will monitor each container, assuring that they keep the required temperature. Finally, once the vaccines arrive to their final destinations at the state and city level, they will need to be stored in ultra-cold freezers. Finally, the Pfizer vaccine will be in the right place and ready to be applied to whoever needs it most.

The Pfizer/BioNTech vaccine is a light of hope, but much work lays ahead
In the end, we have to accept one uncomfortable truth. The immediate return to our normal lives will not be possible, even with the help of this novel vaccine. As mentioned above, the initial supply of this vaccine will be limited. In addition, the first five million doses will be reserved for healthcare workers and people who are at high risk. Consequently, most public health experts agree that Pfizer’s covid-19 vaccine will not reach the general population until mid-2021. Unfortunately, this means that both Americans and Mexicans will need to continue practicing preventive measures such as social distancing, wearing masks, avoiding crowds, practicing hand hygiene and in some cases, getting a coronavirus test. Nevertheless, after more than nine months of this grave pandemic and its fatal consequences, the Pfizer coronavirus vaccine is a light of hope for us all, and the dawn of a new era in vaccine development.
The final goal: stopping the Covid-19 pandemic
To reach “herd immunity” and finally stop the coronavirus pandemic, both the US and Mexico will need to vaccinate at least 80% of its population. To achieve this important goal, an extremely high level of vaccination will be needed. Therefore, the approval of each new coronavirus vaccine brings light and hope, making it possible to reach this milestone.