The Coronavirus Vaccine: an elusive quest and a race against time
The Coronavirus: A World Pandemic
With more than 42 million cases worldwide and more than one million deaths due to Covid-19, this disease has become a world pandemic. Furthermore, country-wide lockdowns aimed to prevent the spread of this virus have placed the world economy at risk.
The Importance of a Coronavirus Vaccine
Considering these facts, people ask and wonder about one important question. How can we return to our normal lives without running the risk of having new coronavirus outbreaks? Unfortunately, quarantines, social distancing, contact tracing, and massive testing programs cannot be the final solution. In reality, these measures can only help to contain the rapid spread of Covid-19. However, they will not be able to reduce its transmission considerably. Thus, the only way to significantly reduce the coronavirus threat is for enough people to become immune to it. Accordingly, once a large percentage of the world population becomes immune to Covid-19, the virus will stop spreading. Consequently, the quest for a coronavirus vaccine has become of utmost importance. In fact, it is the only real and viable way to reach widespread community immunity.
What is a vaccine?
Pathogens are living germs that make you sick. Fortunately, however, scientists can use weakened versions of these pathogens to make vaccines. Therefore, a vaccine will help you develop antibodies that will provide immunity to a certain disease. Best of all, these antibodies will emerge without having to first get sick from the disease.
Scientists are developing several types of coronavirus vaccines
Traditional vaccine types
- Attenuated or inactivated vaccines: Attenuated vaccines are created from a weakened version of the coronavirus. Alternatively, scientists use dead coronavirus particles to create inactivated vaccines. Their main advantage: they are very similar to the disease they help prevent. Thus, they usually create a strong immune response that lasts for many years or even a lifetime.
- Protein-based vaccines: Protein-based vaccines do not contain any genetic material, but instead contain coronavirus proteins. In fact, these vaccines can contain either whole proteins or fragments of proteins. Fortunately, scientists have a good understanding of this protein-based vaccines. In addition, these vaccines have a strong safety record that has been studied and confirmed over the years.
- Repurposed vaccines: vaccines that have been tested and approved for other diseases. In turn, they may also be effective and protect against the SARS-CoV-2 virus.
Novel vaccine types
- Genetic vaccines: genetic vaccines are also known as DNA vaccines. In particular, these vaccines deliver coronavirus genes directly into our cells to create an immune response. Scientists are using Genetic-based immunization as a new approach to vaccine development. DNA vaccines . In addition, some scientists prefer to work with DNA vaccines because they can be produced faster, at a lower cost, and are temperature stable. This makes their storage and transportation much easier.
- Viral vector vaccines: Viral vector vaccines contain viruses. These viruses deliver coronavirus genes to our cells. Once inside, these Covid-19 genes instruct our cells to produce SARS-CoV-2 proteins. In turn, this produces an immune response that will protect individuals from this virus. Advantages: they are easy and relatively cheap to produce. Disadvantages: similar vectors circulate widely (such as the common cold vector). These vectors produce an immune response in some people, making these vaccines ineffective for them.
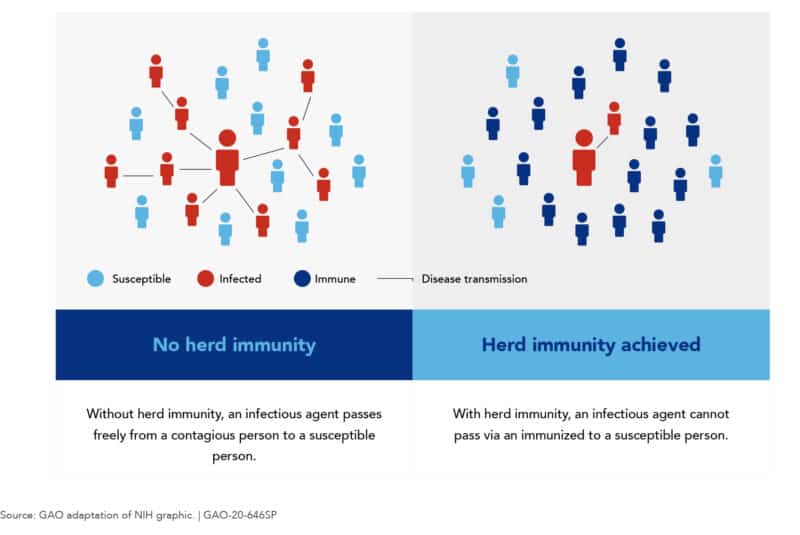
Reaching herd immunity through Covid-19 infections or through a coronavirus vaccine
Most public health experts agree that in order to reach widespread community immunity, 60% or more of the world population would have to become immune to the SARS-CoV-2 virus. Certainly, an important landmark in our fight against Covid-19 is reaching widespread community immunity (also called herd innmunity). Why? Because once this happens, there would be a lot fewer people that the virus could infect. Accordingly, the virus would spread at a much slower pace and we could finally go back to our normal lives. Unfortunately, though, reaching herd immunity is not a simple task. In fact, there are only two ways in which this can be achieved:
Reaching herd immunity through massive Covid-19 infections
Achieving widespread community immunity to Covid-19 through the infection of 60% of the world’s population would mean having 4.5 billion people who would suffer from this grave illness. Needless to say, this would be an unbearable burden for health systems around the world and would lead to millions of deaths. Moreover, the world´s economy and its welfare would be severely affected. Consequently, the only other viable way of achieving herd immunity is through the development and approval of a coronavirus vaccine.
Reaching widespread community immunity through a coronavirus vaccine
The second and most viable option for reaching herd immunity is through the development, approval, and massive deployment of a coronavirus vaccine. Considering that Covid-19 has infected around 4% of the world population, and that these people should be immune to it, two vital tasks remain. These two tasks are developing and approving a Covid-19 vaccine, and deploying the vaccine massively to 4 billion people. Once this happens, we will be able to forget, or at least not be as worried about the coronavirus epidemic that is affecting us all. Accordingly, the following questions become extremely relevant:
- How is a vaccine developed? The coronavirus vaccine development process
- How is a vaccine tested? How are vaccine clinical trials performed?
- What is the probable Covid-19 vaccine timeline?
- When will a coronavirus vaccine be available?
- Who would get a coronavirus vaccine first?
How is a vaccine developed? The coronavirus vaccine development process
The vaccine development process is usually a complex and lengthy undertaking, which takes many years to complete. Most commonly, researchers test and develop vaccines over a ten-year period. Fortunately for all of us, for the coronavirus vaccine development process, scientists are racing to produce a safe and effective vaccine in record-breaking time. These are the most important steps in a vaccine´s development process:
Currently, scientists are testing more than 50 vaccines on humans, in clinical trials. In addition, they are testing at least 100 vaccine prototypes in animal preclinical tests.
Steps to develop a Covid-19 vaccine: from conception to deployment
# 1 – Get to know the pathogen; in this case, get to know the SARS-CoV-2 virus:
The first step in a vaccine´s development process is to know the bug that you are trying to combat. Consequently, you have to be familiar with the structure of a particular germ, because in order to create a vaccine, you basically have to mimic an infection caused by it. In this case, scientists began working on understanding the Covid-19 genome in January of 2020. Fortunately, they quickly deciphered it. Subsequently, with this knowledge, scientists around the world have worked tirelessly to produce weakened versions of it.
Historically, this stage that is also known as the Discovery Research Stage takes anywhere from 2 to 5 years to complete.
# 2 – Developing vaccine prototypes and preclinical testing
The second step of a vaccine´s development process is to create weakened versions of the germ (or the Covid-19 virus). Once scientists manage to do so, they test them on cells, and then on animals such as monkeys. Above all, the purpose of these animal tests is to see if a certain prototype is able to produce an immune response. Thus, preclinical testing is the process in which researchers test these initial vaccines in cells and animals. Finally, after preclinical testing, scientists review their results and decide which compounds should be tested on people.
Usually, preclinical testing takes 2 years to complete. Currently, there are more than 100 compounds in preclinical testing for COVID-19. The companies that are working on preclinical testing are: PittCoVacc/University of Pittsburgh, Sanofi/TranslateBio, Baylor/Biological E, and Novartis/Mass General Hospital.
# 3 – How is a vaccine tested on humans? How are vaccine clinical trials performed?
Once scientists perform preclinical testing in animals, they select several compounds to test them in humans. Therefore, during the clinical trials stage, researchers test different vaccines in humans.
3a – Phase 1 Safety trials for the coronavirus vaccine
During this stage, which is the first stage of clinical trials, researchers give the vaccine to a very small number of people in order to study three things:
- Was the compound safe when used in this small group of people?
- Scientists try to determine the initially suggested dosage that should be used.
- Researchers study the vaccine to see if it produces the desired immune response.
Under normal circumstances, phase 1 of safety trials takes one to two years to complete. Currently, laboratories are testing more than 30 vaccine compounds in Phase 1 of Safety trials. Genexine, Walvax Biotechnology, Inovio, Entos Pharmaceutical, ReiThera, Merck/Institut Pasteur, Vaxart, University of Hong Kong/Xiamen University, DZIF/German Center for Infection Research, ImmunityBio, and Merck/IAVI, are the most important companies that are testing vaccines at this stage.
3b – Phase 2 expanded trials
Once a compound has passed Phase 1 of safety trials, it enters the next stage of clinical trials. Therefore, Phase 2 expanded trials is the secong stage of clinical trials. During this phase, Scientists administer a vaccine to hundreds of people of different age groups. Typically, they administer the vaccine to two distinct groups: children, and elder people. The purpose of this is to see if the vaccine has the same effect in these two groups. In addition, this trial provides further insight into the compound´s safety and its ability to produce an immune response.
Considering past research, phase 2 normally takes two to three years. Currently, several pharmaceutical campnaies are testing more than 20 vaccines in Phase 2 of clinical trials. The most important companies performing tests at this stage are Anges/Osaka University, Imperial College/Morningside, Finlay Vaccine Institute/Soberana1, Arcturus Therapeutics/Duke-NUS Medical School, and Sanofi/Glaxo Smith Kline.
3c – Phase 3 Efficacy Trials
Compounds that finally make it through Phase 2, will start being tested in Phase 3. In reality, however, few vaccines will reach Phase 3 due to the rigorous tests that scientists perform in previous stages. In turn, in Phase 3 scientists administer the vaccine to thousands of people in two groups. First, they administer the vaccine to people in group one. Subsequently, scientists give a placebo to people in group two. Finally, researchers wait to see how many people in groups one and two become infected. Consequently, they try to determine if a vaccine does in fact protect against Covid-19.
Phase 3 efficacy trials take from five to ten years. Currently, there are several research centers that are testing vaccine compunds in Phase 3. The most important companies performing tests in this stage are Moderna/National Institutes of Health, BioNTech/Pfizer, CanSinoBIO/Ad5, Gamaleya/Gam-Covid-Vac, Johnson&Johnson/Adenovirus26, AstraZeneca/Oxford/Covishield, and Novavax.
3d – Vaccine approval / Early or limited approval
Finally, after all of these stages and trials, regulatory agencies in each country review a vaccine’s results. Accordingly, after careful review, they have to decide if they will approve the vaccine or if they will reject its approval. Subsequently, once these agencies have approved and licensed a vaccine, scientists continue to monitor the people who receive it. Most importnatly, they watch and review its effectiveness and safety. Nevertheless, under some extraordinary conditions, regulatory agencies can grant a vaccine “emergency use authorization”. These agencies can grant this special permission before giving its formal and final approval.

What is the probable Covid-19 vaccine timeline?
To talk about a probable Covid-19 vaccine timeline, we have to analyze three factors: research, production, and distribution. Moreover, it is important to understand that these three steps have to be sequential and cannot be performed at the same time. For example, research for the coronavirus vaccine began in January. From that moment on, scientists have tested many vaccine compounds in the different phases of clinical trials. Currently, pharmaceutical companies are testing several promising Covid-19 vaccines in Phase 3 of clinical trials.
If all goes well in the race for a Coronavirus vaccine…
In a best-case scenario, these vaccine compounds will complete their phase 3 trials by the end of November of 2020. If this happens, full and clear information about its results in phase 3 trials will probably be available by the beginning of 2021. Finally, if one of these vaccine compounds has successful results in phase 3 trials and after careful review, regulatory agencies would grant its approval. Therefore, coronavirus vaccine production will probably begin in April of 2021 or even later. Finally, as most experts predict, in an overly optimistic scenario, a coronavirus vaccine would be ready for distribution probably by August of 2021.
Probable Covid-19 timeline in a best-case scenario
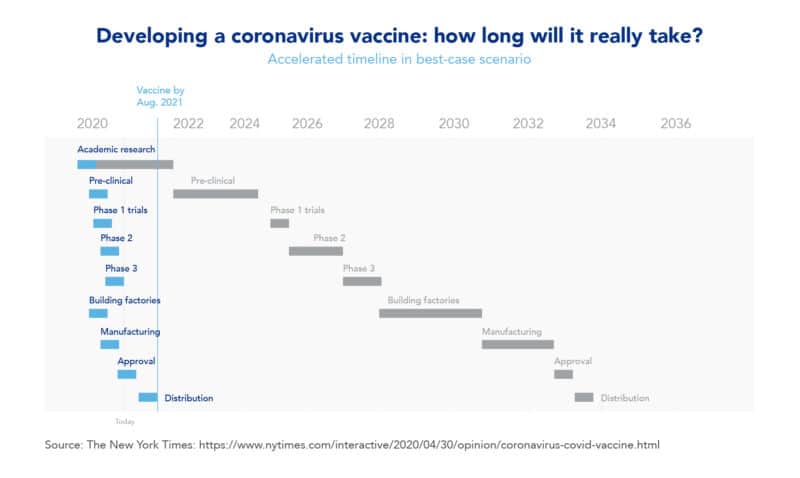
In this example of a best-case scenario coronavirus vaccine timeline, many of its stages would be performed simultaneously to expedite the process. In reality, this is what it’s actually happening in the race for developing a coronavirus vaccine. In this case, research begun in January of 2020. Subsequently, in March of 2020, researchers performed pre-clinical testing. Then came Phase 1 and Phase 2 of clinical trials, which scientists have performed since May of 2020, and in some cases even simultaneously. Currently, several renowned research centers are testing more than five vaccines already in Phase 3. Finally, if everything goes well, these companies will complete manufacturing and approval by April or May of 2021, and will distribute the vaccine in August of 2021.
Example of a typical vaccine development timeline
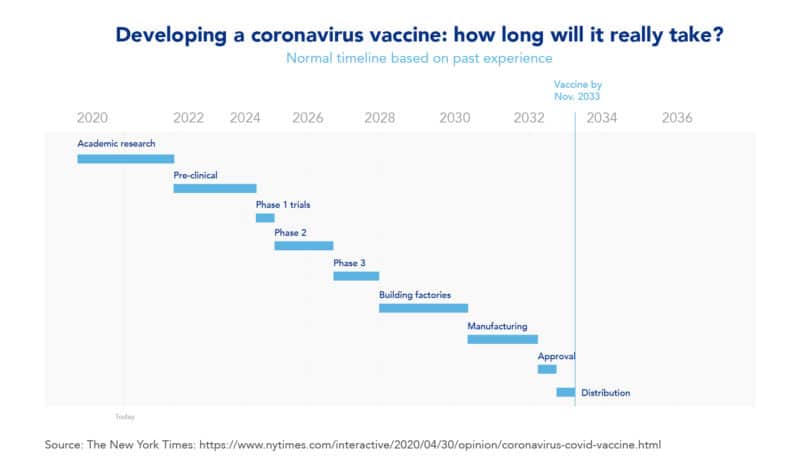
In this example of a typical vaccine development timeline, early vaccine research for a newly discovered bug, like the coronavirus, would begin in January of 2020. In this typical timeline, the academic research stage would take from 2020 to 2022 and the pre-clinical stage would take from 2022 to 2024. Subsequently, its clinical trials, from Phase 1 to Phase 3, would take from 2024 to 2028, and production and approval would go from 2028 to 2032. Finally, the distribution and deployment of the vaccine would be performed in 2034.
When will a coronavirus vaccine be available?
As the Covid-19 virus continues to spread and claim lives, people around the world want to know when will a coronavirus vaccine be available. In turn, some politicians have said that several Covid-19 vaccines could be available by the end of 2020 if all goes well. Nevertheless, it is important to consider that most of the vaccines that exist today took years of testing before actually reaching the general population. In fact, as we can observe in the table below, scientists developed several of today’s most common vaccines over a ten to twenty-year period.
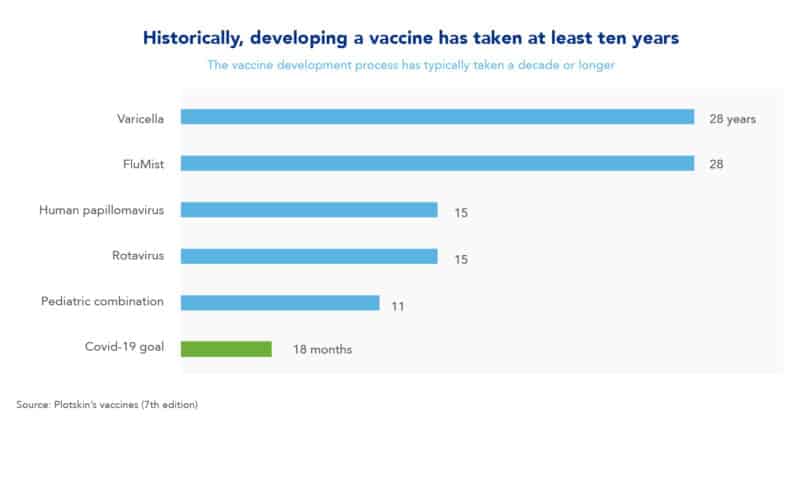
The pediatric combination vaccine: an eleven year development example
Here are three examples of vaccine develpoment: the pediatric combination vaccine took eleven years to develop, and the rotavirus and human papillomavirus vaccines took fifteen years to develop. As we can observe with this past record, the goal of getting a coronavirus vaccine developed and approved in just eighteen months seems extremely ambitious. Nevertheless, if everything goes as planned and in a best-case scenario, it could be possible to have a Covid-19 vaccine ready probably by August of 2021. On the other hand, in a more normal and less optimistic scenario, experts estimate that a coronavirus vaccine could be ready in 2023.
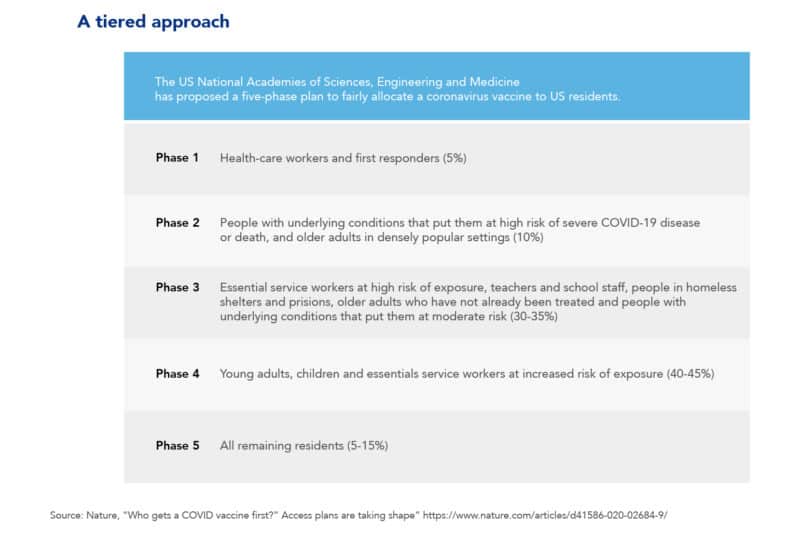
Who would get a coronavirus vaccine first?
Whether developing and approving a coronavirus vaccine takes months or years, one thing is for certain: initially, it will be in short supply. Therefore, as the coronavirus continues to spread, a relevant question is “Who should get vaccinated first?”. Accordingly, to answer this question, the international community created two advisory groups. One was created at the World Health Organization (WHO) and another at the US National Academies of Sciences, Engineering, and Medicine (NASEM). In turn, these two groups have proposed a five-phase plan for administering the vaccine. The goal of this plan is to prioritize and fairly allocate the Covid-19 vaccine among population groups. For example, the table below suggests that the first group that would get the vaccine would be Health-care workers. Subsequently, the second group that would be vaccinated is that of people with underlying conditions that put them at risk of a severe illness or death.
In conclusion:
Developing, approving, and manufacturing a coronavirus vaccine is of vital importance. Moreover, having it ready and deploying it as fast as possible is paramount. In turn, only time will tell if scientists are able to develop a Covid-19 vaccine in record-breaking time. Hopefully, this amazing undertaking will be succesful, making it possible for the world to have a coronavirus vaccine by August of 2021.
Frequently asked questions about a coronavirus vaccine
Unfortunately, regulatory agencies have not approved a coronavirus vaccine for use with the general population. However, Russian and Chinese agencies have approved two viral vector vaccines for limited/early use in these countries.
Covid-19 vaccine approved for limited use in China
CanSinoBIO, viral vector vaccine: this coronavirus vaccine has been approved for limited use in China. Accordingly, scientists are currently administering this vaccine among chinese soldiers. CanSinoBIO was approved on June 25th of 2020 by Chinese military leaders.
Covid-19 vaccine approved for limited use in Russia
Gam-Covid-Vac/Sputnik V, viral vector vaccine: This vaccine was developed by Russia’s Ministry of Health. In The Gamaleya Research Institute. This Russian vaccine is a combination of two adenoviruses that scientists engineered with Covid-19 genes. Above all, this vaccine became widely known because Russian healthcare regulators approved it even before it reached Phase 3 trials. Currently, Russian researchers have administered the vaccine to more than 40,000 Russian volunteers. In addition, the Russian government has already negotiated agreements to supply the vaccine to several countries, including Mexico.
Vaccines get approved through a rigorous and lengthy process in which vaccine compounds tested by going through different phases. Typically, a vaccine’s approval process consists of the following stages: discovery/exploratory stage, pre-clinical stage, clinical trials, regulatory review and approval, manufacturing, and quality control.
Although it is still uncertain, experts believe that there is a good chance that the flu shot could provide some protection against Covid-19. For example, according to virologist Roberto Gallo from the University Of Maryland School Of Medicine: the flu vaccine could offer some protection against coronavirus. However, a key factor for this to have a chance of working is getting the right type of flu vaccine. Accordingly, his recommendation is to get a flu vaccine that has a live virus in it. Most commonly called a “live attenuated vaccine”. This is important because scientists have discovered over years of experience, that live attenuated vaccines seem to offer some protection not only against the disease that they were made for but also against other diseases (including respiratory diseases such as the coronavirus).
In general, experts believe that the pneumonia vaccine does not provide protection against the SARS-CoV-2 virus. Nevertheless, although the pneumonia vaccine may not provide some protection against Covid-19, it is recommended to protect your health.
Following the typical clinical trial timeline, Phase 3 clinical trials take at least three years to complete. However, in most cases, Phase 3 clinical trials take a bit longer, usually ranging from five to ten years. Nevertheless, for the first time in history scientists are working against the clock trying to develop a coronavirus vaccine. Therefore, pharmaceutical companies and research centers are trying to complete the Phase 3 trial stage in three to six months.